Choosing the right equipment for outdoor activities is essential to ensure a safe, positive, and transformative experience. This is particularly important when it comes to outdoor cooking gear. Factors such as the biome, time of year, weather conditions, group size, fuel availability, and even local regulations must be considered when deciding the right cooking system, which includes stoves, fuels, pots, and utensils.
When I go trekking and need to cook outdoors, I usually opt for gas stoves due to their practicality and ease of use. Although there are options such as alcohol stoves (spirit burners) and stoves that use white gas, gasoline, or other petroleum derivatives, gas stoves are the most common choice for most people. However, the efficiency of a gas stove can be significantly affected by the composition of the gas, especially in low-temperature conditions.

In this article, I will explore how different types of gas, such as butane, propane, and isobutane, behave at various temperatures. In addition, I will discuss the best gas mixture options to ensure your stove’s efficiency in different scenarios, providing a safe and effective outdoor cooking experience regardless of the weather conditions.
Gas composition and boiling point
The boiling point of the gas is crucial to its performance in low temperatures. Boiling is the process by which a liquid turns into gas (vapor). For the gas stored in liquid form inside the canister to vaporize and be burned in the stove, it needs to reach its boiling point. If the ambient temperature is below the gas’s boiling point, the liquid does not vaporize efficiently, resulting in lower pressure and a weaker flame.
- Butane: boiling point of approximately -1°C.
- Isobutane: with a boiling point of approximately -11.7°C, isobutane is more efficient in cold temperatures compared to butane.
- Propane: Propane has a very low boiling point of -42°C, making it highly efficient in freezing conditions. However, its high vapor pressure requires very robust and heavy canisters, which may not be practical for trekking. Propane canisters do exist, but they are typically used with larger stoves for family camping.

The boiling point of the gas is the temperature at which it changes from liquid to gas, but stove efficiency can begin to fail at higher temperatures due to evaporative cooling, which lowers the canister’s temperature during prolonged use, decreasing internal pressure and efficiency. For example, butane, with a boiling point of -1°C, begins to fail at temperatures of 5°C or lower, while isobutane, with a boiling point of -11.7°C, loses efficiency around 0°C due to these combined effects.
To minimize the effects of temperature and the pressure required to liquefy propane, the gas canisters used in trekking stoves usually contain a gas mixture rather than a single type of gas.
Gas mixtures
The solution to maintaining stove efficiency in cold climates is to use gas mixtures. The best composition is between 20% to 30% propane and 80% to 70% isobutane. This mixture provides efficient burning at low temperatures, but even so, in extreme cold conditions, there may be a drop in efficiency.
In the table below, I show some gas canister manufacturers with their respective compositions.
\[ninja\_tables id=”25498″]
\* n.i.: composition not provided by the manufacturer. Only the gases are specified, but not the percentage of each one.
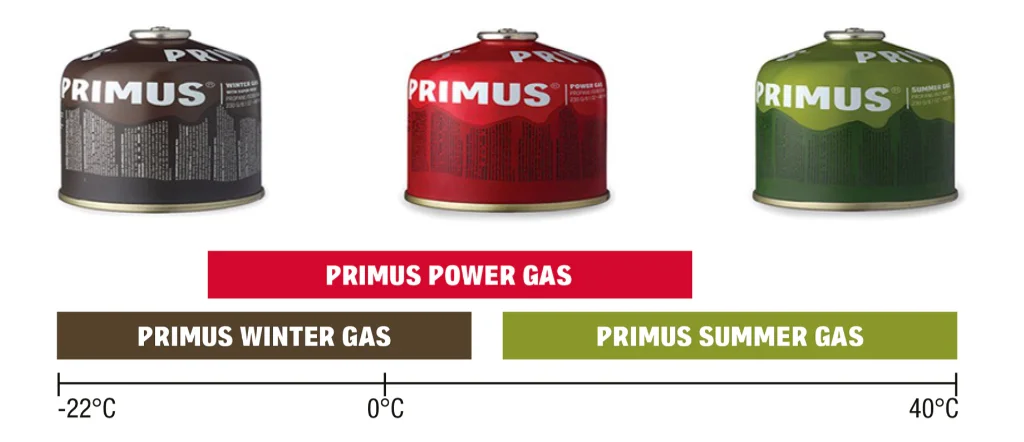
Some manufacturers offer more than one type of gas, with different compositions or technologies, to suit different temperatures. Decathlon, for example, increases the propane percentage from 30% to 40%, improving stove efficiency in low temperatures. Primus, a Swedish brand founded in 1892 and specialized in stoves, created an innovative technology in its Winter Gas canisters that, with the same gas composition, enhances performance at lower temperatures. This is because they included a technology called VaporMesh in the canister, which makes gas vaporization more efficient. On the other hand, the brand also developed another gas mixture, Summer Gas, to meet the needs of trekkers or campers in warmer climates. It is worth noting that variations in mixtures and technologies make the cost of canisters higher or lower.
The illustration below shows the operating temperature ranges for the 3 different types of Primus canisters.
How gas stoves work
Gas stoves work by vaporizing the liquefied gas contained in the canisters. The gas is stored in liquid form due to the high pressure inside the canister. When the stove valve is opened, the pressure is reduced, allowing the gas to vaporize and be burned to generate heat.

At low temperatures, vaporization can be less efficient due to the boiling point of the gas. As explained earlier, gases such as butane, which have a higher boiling point, may not vaporize properly, reducing stove performance.
This situation occurs in upright stoves, where fuel vaporization happens at the valve outlet. However, there is a type of stove used in expeditions that helps solve this issue, even when using a fuel with less propane in cold environments. But before talking about this, let’s physically understand what happens with gases and why they are affected by temperature.
Ideal Gas Law
To better understand how gas composition impacts stove performance, it is important to consider the Ideal Gas Law, which relates pressure (P), volume (V), and temperature (T) of a gas. The equation is expressed as:
Where:
P is the gas pressure.
V is the volume occupied by the gas.
n is the number of moles of the gas.
R is the universal gas constant (8.314 J/mol·K).
T is the temperature in Kelvin (K).
The Ideal Gas Law combines Boyle’s, Charles’s, and Avogadro’s laws into a single equation:
Boyle’s Law: For a fixed amount of gas at constant temperature, volume is inversely proportional to pressure (PV = k).
Charles’s Law: For a fixed amount of gas at constant pressure, volume is directly proportional to absolute temperature (V ∝ T).
Avogadro’s Law: Equal volumes of all gases, at the same temperature and pressure, contain the same number of molecules (V ∝ n).
Effects of temperature on stove performance
Pressure and Temperature
According to the Ideal Gas Law, the pressure of the gas inside the canister is directly proportional to the temperature. This means that when the ambient temperature decreases, the pressure inside the canister also decreases, resulting in lower stove efficiency. At low temperatures, the lower pressure may not be sufficient to vaporize the gas efficiently, causing a weaker flame or even stove failure.
Test between Primus Power Gas and Winter Gas canisters
When Primus compared two completely new and full gas canisters (Power Gas and Winter Gas), the difference was not noticeable initially, as the pressure was high in both canisters. However, as more gas was consumed, the difference became more evident.
When gas canisters without VaporMesh (Primus Power Gas) are used in cold conditions, the pressure inside the container decreases due to the cold, which means the stove burns with less power or may even stop working. This becomes more obvious when the container is no longer full. After 60 minutes of cooking, Primus Winter Gas is about 9% more powerful than a gas canister without VaporMesh. After 120 minutes of use, it delivers about 15% more power.
Check out the table of a comparative test between a full Power Gas canister and a full Winter Gas canister, carried out in a cold chamber with a constant temperature of -20 °C. Both gas canisters were left in the freezer for 48 hours before the test was conducted to better reflect a real situation when you are outdoors for several days. The test used two Primus Spider stoves and an infrared thermometer with a laser pointer.
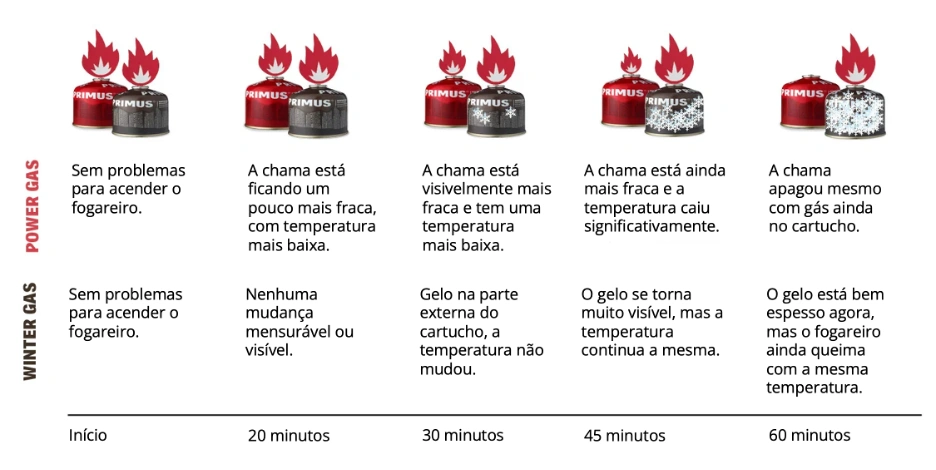
* test conducted by Primus. The information was taken from https://primusequipment.com/en-eu/pages/primus-winter-gas
Evaporative cooling
Another important phenomenon to consider is evaporative cooling. When liquefied gas evaporates, it absorbs heat from the environment and from the canister itself, causing the canister temperature to drop. This additional cooling can further reduce internal pressure, exacerbating performance problems in low temperatures.
What happens to the gas canister in practice?
- Heat Absorption by the Gas: When we light the stove and the gas begins to evaporate, it absorbs heat from the environment and the canister. This heat absorption process is essential for the gas to change from liquid to gaseous state.
- Drop in Canister Temperature: As the gas continues to evaporate, it draws more heat from the canister, causing the canister temperature to decrease. This effect is particularly noticeable when the stove is running continuously for long periods. It becomes cold and, depending on relative humidity, it will become wet due to condensation.
- Decrease in Internal Pressure: With the drop in canister temperature, the internal pressure also decreases. The Ideal Gas Law tells us that the pressure of a gas is directly proportional to its temperature (P ∝ T). Therefore, when the temperature drops, the pressure inside the canister also drops.
- Reduction in Stove Efficiency: The lower internal pressure means there is less force to “push” the gas out of the canister and into the stove burner. This can result in a weaker and less efficient flame. In extreme cases, the pressure may drop so much that the stove may stop working, even if there is still gas in the canister.
Strategies to mitigate evaporative cooling
To minimize the effects of evaporative cooling and ensure more consistent stove performance, you can adopt a few strategies:
- Warm up the canister: Keep the gas canister warm before using it. You can do this by keeping it close to your body.
- Choose gases suitable for the climate: As explained earlier, use gas mixtures appropriate for low temperatures, such as propane and isobutane blends, which have lower boiling points and better performance in cold conditions.
- Use stoves with remote canisters: Some stoves allow the canister to be used in an inverted position, which helps maintain pressure and improve efficiency in cold temperatures.
Stoves with remote canisters
Stoves with remote canisters can be a solution to improve cooking system efficiency. Unlike upright stoves, where the burner sits on top of the gas canister, remote canister stoves feature a flexible hose, allowing the canister to be placed away from the stove.

However, not every stove that uses the canister remotely will work in this case. Only those with a fuel preheat tube, located under the burner, will function properly.
The preheat tube is used to vaporize the fuel that reaches it in liquid form, and it does so thanks to the heat of the stove’s own flame. This is how white gas and other liquid-fuel stoves manage to vaporize fuel. In the case of gas canisters, when used in an inverted position (gas canister upside down), they allow the fuel to leave still in liquid form and be vaporized in the preheat tube.
Pressure required for gas liquefaction
The pressure required to liquefy gases varies, and when we talk about a gas mixture, such as those found in stove canisters, it ends up being proportional to the concentration of the different gases in the mixture. See below the vapor pressure for the liquefaction of each gas mentioned in the article:
- Butane: Vapor pressure at 25°C is approximately 2.1 bar.
- Isobutane: Vapor pressure at 25°C is about 3.1 bar.
- Propane: Vapor pressure at 25°C is around 8.4 bar.
Although propane is ideal for extreme cold conditions due to its low boiling point, the high pressure required to keep it in the liquid state demands canisters with much thicker and more robust walls. This makes the canisters heavier, which may be impractical for trekking and other outdoor activities where weight is a crucial factor. The canister shown as an example is used in family camping stoves and, due to this type of activity, the extra weight and volume are not an obstacle.

Use of gas stoves in Brazil
The NTK TekGás 230 g gas canister, which contains a mixture of 75% isobutane and 25% propane, is an excellent choice for conditions found in Brazil, both in summer and winter. This combination offers reliable performance across a wide temperature range, ensuring efficiency even in intense cold situations, where temperatures can easily reach -5 ºC. In summer, isobutane provides stable and efficient burning, while propane ensures that the stove works properly in lower temperatures, making the NTK TekGás 230 g a versatile and practical option for Brazilian adventurers throughout all seasons of the year.
Final considerations
When planning an outdoor activity, especially in cold climates, choosing the right gas canister is crucial. Opting for isobutane and propane mixtures can ensure that your stove works efficiently, even in adverse temperatures. Knowing the characteristics of each type of gas and how they behave in different conditions will allow for a safer and more effective outdoor cooking experience.
Don’t forget that planning is essential. In this article, we focused on gas canisters, but it is important to take into account the cooking system as a whole. This includes deciding the right fuel, stove, and cookware, considering group size or individual use, fuel availability, ambient temperature, and other weather conditions. Careful planning and correct equipment selection will ensure that you have a safe and successful outdoor experience.
This post is also available in: Português (Portuguese (Brazil)) Español (Spanish)
